Biodiesel Fuel Quality
|
|
|---|
Introduction
For biodiesel to be sold in the market, the fuel must meet certain quality specifications. In the United States, biodiesel must meet the American Society for Testing and Materials requirements for biodiesel fuel in its D 6751 standard. The standard in Europe is defined by EN14214.
Using biodiesel that does not meet the quality specifications may have consequences as severe as engine seizure, filter plugging, and adverse tailpipe emissions.
This article will deal with ASTM D6751. The quality specifications mentioned in this article are for 100% pure biodiesel, commonly known as B100, unless otherwise specified. The specifications cover biodiesel (B100) for use as a blend component with diesel fuel defined by Specification D 975 Grades 1-D, 2-D, and low sulfur 1-D and 2-D ASTM (2003). The definition of biodiesel according to this standard is “a fuel comprised of mono-alkyl esters of long chain fatty acids derived from vegetable oils or animal fats, designated B 100.”
The first biodiesel quality standard was adopted in 2002 as ASTM D6751-02. After publication of the first standards, several parameters were added and/or modified. The most recent version of the ASTM specification is D6751-09 (Table 1).
Table 1: The ASTM D6751-09 specification. The quality specifications that are modified from original ASTM D6751-02 are in bold; new specifications added over time are marked with an asterisk. Source: ASTM (2009); NBB (2009)
| Property | ASTM Method | Limits | Units |
| Flashpoint | D93 | 93.0 Min | °C |
| Water & Sediment | D2709 | 0.050 Max | % Vol. |
| Kinematic Viscosity, 4O°C | D445 | 1.9-6.0 | mm2/Sec. |
| Sulfated Ash | D874 | 0.020 Max | % Mass. |
| Sulfur | |||
| S 15 Grade | D 5453 | 0.0015 max. | % mass (ppm) |
| S 500 Grade | D 5453 | 0.05 max. | % mass (ppm) |
| Copper Strip corrosion | D130 | No. 3 Max | |
| Cetane | D613 | 47 Min | |
| Cloud point | D2500 | Report | °C |
| Carbon Residue | D4530 | 0.050 Max | % Mass |
| Acid Number | D664 | 0.50 Max | mg KOH/g |
| Free Glycerine | D6584 | 0.020 Max | % Mass. |
| Total Glycerine | D6584 | 0.24 Max | % Mass. |
| *Calcium & Magnesium combined | EN 14538 | 5 Max | ppm (µg/g) |
| *Alcohol Control (one of the following must be met) | |||
| 1. Methanol Content | EN14110 | 0.2 Max | % volume |
| 2. Flash Point | D93 | 130 Min | °C |
| *Phosphorus Content | D 4951 | 0.001 Max | % mass |
| *Distillation Temperature Atmospheric equivalent temperature, 90 % recovered |
D 1160 | 360 Max | °C |
| *Sodium/Potassium combined | EN 14538 | 5 Max | ppm |
| *Oxidation Stability | EN 14112 | 3 minimum | hours |
| *Cold Soak Filtration For use in temperatures below -12°C |
Annex to D6751 Annex to D6751 |
360 Max 360 Max |
seconds seconds |
Specifications Demystified
The properties of biodiesel depend on several factors, including the feedstock and the refining process. Producers who follow standard procedures to make the fuel, as described in Biodiesel Production Principles and Processes, will have a better chance of producing fuel that meets the specifications.
The following is a brief description of each quality parameter, why it is important, how fuel is tested for this specification, and what producers can do if a batch of fuel is out of spec.
Flash Point
The flash point of a fuel is the lowest temperature at which its vapor can be ignited. The flash point is not directly related to engine performance. It is, however, of importance in connection with legal requirements and safety precautions involved in fuel handling and storage. The flash point for biodiesel has been set at 93°C (200°F) minimum, so biodiesel falls under the non-hazardous category of the National Fire Protection Association codes.
Compared to biodiesel, diesel has a much lower flash point requirement of 52°C as specified in ASTM D975. In that regard, biodiesel that meets the ASTM specification is safer to handle than regular diesel. The flash point of pure biodiesel is generally over 150°C – much greater than the specification requires. A lower flash point might mean there is methanol left over in biodiesel. If a batch of biodiesel does not meet the flash point standard, separating the methanol from the biodiesel should increase the flash point above the minimum requirement.
Water and Sediment
Biodiesel should be dried after water washing to get the water specification below 500 ppm (0.050%). Water in biodiesel could make the fuel go rancid and alter the chemical structure of biodiesel. If moisture is allowed to accumulate for a long time, it will increase the free fatty acid level of biodiesel. Free fatty acids may corrode metal parts in fuel lines. They can also react to make monoglycerides.
Unfortunately, even when biodiesel is dried adequately by the producer, water can accumulate during transportation and storage. Compared to regular diesel, biodiesel is more hygroscopic – it attracts water – due to its polar molecular structure at one end. He et al. (2007) found that biodiesel absorbed 1,000 to 1,700 ppm (0.10% to 0.17%) moisture at temperatures of 4°C to 35°C, which was 15 to 25 times higher than that of D2 in the same temperature range. As the temperature increased, moisture increased at a rate of 22.2 ppm/°C, which was more than nine times higher than that of diesel.
This may lead to a phenomenon in which stored biodiesel absorbs water at high temperatures, which then precipitates out when the temperature drops. The process may repeat, and water accumulation could occur at the bottom of storage vessels.
The best way to deal with water in biodiesel is to dry the fuel adequately and then use the fuel quickly – within a few months – to prevent water from accumulating. If biodiesel is stored for more than a few months, the fuel should be checked for moisture content before use.
The water and sediment specification is also concerned with any substance that is higher in density than biodiesel, such as monoglycerides and unreacted oil. Like water, these heavier substances can settle in the tank. Water in biodiesel storage tanks can promote algae growth that can clog fuel filters when transferred to vehicle and equipment tanks.
To measure the water and sediment, a 100-mL sample of undiluted fuel is centrifuged at a relative centrifugal force of 800 for 10 minutes at 21° to 32°C (70° to 90°F). After centrifugation, the volume of water and sediment which has settled into the tip of the centrifuge tube is read to the nearest 0.005 mL and reported as the volumetric percent of water and sediment.
Kinematic Viscosity
Kinematic viscosity measures the ease with which a fluid will flow under force. It is different from absolute viscosity, also called dynamic viscosity. Kinematic viscosity is obtained by dividing the dynamic viscosity by the density of the fluid. If two fluids with the same absolute viscosity are allowed to flow freely on a slope, the fluid with higher density will flow faster because it is heavier.Viscosity refers to a fluid’s resistance to flow at a given temperature. A fuel that is too viscous can hinder the operation of an engine.
The density of biodiesel varies depending on its feedstock. Longer and straighter chains (saturated fats) tend to have higher density than shorter and unsaturated molecules. Kinematic viscosity allows comparison between the engine performance of different fuels, independent of the density of the fuels. Two fuels with the same kinematic viscosity should have the same fuel properties, even though one fuel may be denser than the other.
The highest acceptable kinematic viscosity for biodiesel as specified in D6751 is 6.0. EN 14214, the biodiesel standard for the European market, specifies a viscosity limit for biodiesel of 3.5 to 5.0 mm 2/s. If a batch of biodiesel does not meet this specification, the viscosity can be corrected by blending it with a fuel that has a lower or higher viscosity.
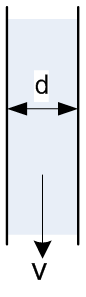
For those who would like to do the calculations themselves, Figure 1 shows the equation for the velocity of fluid flowing at the center of the pipe:
Where, ρ is the density of the fluid, g is gravity, d is the pipe diameter and µ is the fluid viscosity. When everything else is constant, the fluid with higher density will flow faster than the fluid with lower density. Therefore, to obtain a measure of ease of flow under gravity that is density independent, the above equation is divided by density and defined as kinematic viscosity. Therefore, the kinematic viscosity for this example would be
Substituting for µ in above equation, we get:
Therefore, this equation ensures that if two fluids have the same kinematic viscosity, their flow property will be similar regardless of the kind of fluid.
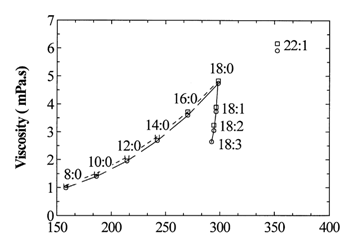
Figure 2: Viscosity trend lines for methyl ester and ethyl ester GC standards at 40°C: (□) EEs and (O) MEs. (Source: Allen et al. (1999))
The viscosity of a mixture of different types of biodiesel can be estimated using the equation (Allen et al. (1999); Tat and Van Gerpen (1999))
- Log(v) = m1Log(v1) + m2Log(v2)
Where ν is the viscosity of the mixture, m1 and m2 and mass fractions of two biodiesels being mixed, and ν1 and ν2 are their respective viscosities.
Sulfated Ash
Sulfated ash is a measure of ash formed from inorganic metallic compounds.
When biodiesel burns, theoretically it should produce only CO2 and water. However, in practice, biodiesel leaves some ash from unburned hydrocarbon and from inorganic impurities. The ash-forming inorganic materials may be present in biodiesel in three forms: (1) abrasive solids, (2) unremoved catalysts, and (3) soluble metallic soaps.
Metal-containing fuel additives and unremoved catalysts are the main contributors of sulfated ash. Abrasive solids contribute to injector, fuel pump, piston and ring wear, and engine deposits. Soluble metallic soaps have little effect on wear but may contribute to filter plugging and engine deposits (ASTM, 2009).
To measure sulfated ash, biodiesel is burned and the ash is collected. Burning leaves metal oxides (metallic ash) and unburned hydrocarbon. The ash is then treated with sulfuric acid and heated to 775°C (1427°F). This completely oxidizes the carbon residue, which evaporates as CO2. Any metallic oxide (such as calcium oxide) is transformed into metallic sulfate, such as calcium sulfate. What remains after completely drying is reported as sulfated ash.The damage caused by ash from unburned hydrocarbon is different from the damage caused by metallic ash. Metallic ash is abrasive and can cause serious damage to the interface between the piston ring and the cylinder wall. Therefore, sulfated ash should be measured separately from residue carbon.
Sulfur
In 2006, the United States Environmental Protection Agency mandated that the sulfur level of on-highway diesel fuel be reduced from 500 ppm to 15 ppm. Biodiesel and its blends with fossil diesel must comply with this mandate as well.
Biodiesel made from virgin soybean oil does not contain any sulfur since soybean oil does not contain sulfur. Canola, rapeseed, and mustard contain varying amounts of glucosinolates, which are sulfur-containing compounds. Canola, which has been bred to be low in glucosinolates, contains sulfur fatty acids. It is the only edible oil known to contain these compounds, and as a result, canola oil contains from 3 to 25 ppm of sulfur (Gunstone, 2004). Some used vegetable oil, especially oil that has been used to cook sulfur-rich foods such as onion rings, may also have a higher than 15 ppm level of sulfur. Biodiesel made from high sulfur oil may also contain a high level of sulfur.
Total sulfur analysis is performed using ultraviolet fluorescence. Biodiesel that is high in sulfur could be treated with Magnesol, a product from Dallas Group that removes sulfur.
Ultra-low sulfur diesel lacks sufficient lubricant characteristics to adequately lubricate diesel engine fuel pumps. Many wholesale blenders add up to 2% biodiesel to increase the lubricity characteristics of the fuel blend.
Copper Strip Corrosion
This test serves as a measure of possible difficulties with copper, brass, or bronze parts of the fuel system.
A polished copper strip is immersed in 30mL of biodiesel at 50°C for three hours. After the test period, the strip is examined for evidence of corrosion, and a classification number from 1 to 4 is assigned based on a comparison with the ASTM Copper Strip Corrosion Standards. The presence of acids or sulfur-containing compounds can tarnish the copper strip, thus indicating the possibility for corrosion of engine parts (ASTM, 2009).
For biodiesel, the copper strip corrosion test value should be as low as 1. High copper strip corrosion indicates a severely degraded or acid-contaminated fuel.
Cetane Number
The cetane number (CN) indicates how well a fuel will combust inside a compression engine. Biodiesel usually has a higher cetane number than petroleum diesel. The CN of biodiesel varies from 45 to 67 (Van Gerpen, 2006).
The cetane number of pure biodiesel depends on its fatty acid profile. Biodiesel from saturated fat will have a higher cetane number than biodiesel from unsaturated oil. Therefore, the cetane number of a biodiesel can be improved by adding biodiesel from a feedstock higher in saturated fat. However, it should be kept in mind that biodiesel from saturated fats tends to have a higher cloud point and will gel at higher temperatures than biodiesel made from unsaturated oil.
Cloud Point
Biodiesel tends to freeze at higher temperatures than petro-diesel. This is one of the major factors hindering the use of biodiesel. The cloud point (CP) is the temperature of the fuel at which small, solid crystals can be observed as the fuel cools. The cloud point is well correlated with the filter plugging point, which occurs when the fuel begins to clog filters and hinder the operation of the engine.
The ASTM D6751 standard does not specify a cloud point for biodiesel. However, the cloud point must be tested and revealed to the buyer.
The ASTM D 2500-02 specification is used to test the CP of all blends of biodiesel fuel. The specimen of fuel is cooled at a specified rate and examined periodically. The temperature at which a cloud is first observed at the bottom of the test jar is recorded to the nearest 1°C as the cloud point.
The cloud point depends mostly on the fatty acid profile of the feedstock and the type and quantity of impurities in the fuel. Impurities such as monoglycerides can greatly increase the cloud point. Biodiesel made from saturated fats has a higher cloud point than biodiesel made from unsaturated fat.
Adding cold flow additives does not reduce the CP of biodiesel very much. Shrestha et al. (2008) found that the average reduction of cloud point from several commonly available cold flow additives on B100 methyl ester was 0.6°C.
Biodiesel can be mixed with petro-diesel to lower its cloud point during cold weather.
Carbon Residue
Carbon residue gives an approximate measure of the carbon-depositing tendencies of a fuel oil.
A weighed fuel sample is placed in a glass vial and heated to 500°C under an inert (nitrogen) atmosphere in a controlled manner for a specific time. The sample undergoes coking reactions, and volatiles formed are swept away by the nitrogen. The carbonaceous-type residue remaining is reported as a percentage of the original sample as “carbon residue.”
Acid Number
The acid number indicates the acidity of the fuel. The test measures the amount of potassium hydroxide needed to neutralize 1 gram of fuel.
Pure biodiesel is not acidic. However, after biodiesel is washed with acidic water to neutralize the catalyst, the fuel may have some residual acid. In addition, biodiesel can absorb water during storage, which can lead to the formation of free fatty acids.
Fuel with a high acid value has a greater tendency to corrode fuel tank, linings, and pipelines. This parameter can also be used as a measure of the freshness of the fuel. Fuel that has oxidized after long-term storage will probably have a higher acid value.
Free and Total Glycerin
Free and total glycerin in biodiesel affects the fuel quality in several ways. Fuel with excessive free glycerin usually causes problems with glycerin settling down in storage tanks. This creates a viscous mixture that can plug fuel filters and create combustion problems in engines (Van Gerpen, 2008).
Residual glycerides in the fuel are caused by an incomplete transesterification reaction. According to the ASTM D 6751 standard, biodiesel should contain a maximum of 0.02% of free glycerol (FG) and a maximum of 0.24% total glycerol by weight.
Calcium and Magnesium
This specification was introduced in 2006 and addresses the potential effects of small levels of calcium and magnesium on diesel particulate traps. Calcium and magnesium compounds may be collected in exhaust particulate removal devices and are not typically removed during passive or active regeneration. This can create increased back pressure and reduced time to service maintenance (ASTM, 2009).
Calcium and magnesium can be introduced during the biodiesel production process – for example, through the use of calcium methoxide as a solid base catalyst (Liu et al., 2008). Producers must be careful not to leave any calcium residue in biodiesel.
Calcium and magnesium may be present in biodiesel as abrasive solids or soluble metallic soaps. Abrasive solids can contribute to injector, fuel pump, piston and ring wear as well as to engine deposits. Soluble metallic soaps have little effect on wear, but they may contribute to filter plugging and engine deposits. The presence of these metals also contributes to the sulfated ash measurement.
Phosphorus Content
Because phosphorus can damage catalytic converters used in emissions control systems, its level must be kept low. Catalytic converters are becoming more common on diesel-powered equipment as emissions standards are tightened, so low phosphorus levels will be of increasing importance. Biodiesel produced from U.S. sources has been shown to have a low phosphorus content (below 1 ppm), and the specification value of 10 ppm maximum is not problematic. Biodiesel from other sources may or may not contain higher levels of phosphorus. This specification was added to ensure that all biodiesel, regardless of the source, has low phosphorus content (ASTM, 2009).
Sodium and Potassium
Sodium and potassium, like calcium and magnesium, may be present in biodiesel as abrasive solids or soluble metallic soaps. This specification is intended for a very similar reason as calcium and magnesium.
Sodium and potassium in biodiesel usually come from the catalyst not being removed after the reaction through water washing or other process. Use of catalyst in excess may result in having an off-spec biodiesel. If water is present during biodiesel reaction, some soap is formed and usually remains dissolved in biodiesel. That also gives rise to a high level of sodium or potassium salt.
Oxidative Stability
The oxidation of biodiesel can produce various acids or polymers, which, if in high enough concentration, can cause fuel system deposits and lead to filter clogging and fuel system malfunctions. Additives designed to retard the formation of acids and polymers can significantly improve the oxidation stability performance of biodiesel (ASTM, 2009).
Cold Soak Filtration
This requirement was added in 2008 in response to data indicating that some B100 could, in blends with petroleum diesel of up to 20%, form precipitates above the cloud point. These precipitates can clog filters. The cold soak filtration test determines if biodiesel shows precipitate formation upon cooling to temperatures above the cloud point.
The test is performed by placing 300 mL of biodiesel in a 500-mL glass bottle. The sample is then set in a liquid or air bath or chamber at 40°F for 16 hours. The sample is then allowed to come to room temperature (around 70°F) and filtered through a 47-mm diameter and 0.7-μm nominal pore-sized glass fiber filter under vacuum of 25 inches of mercury under atmospheric pressure. The time required to filter 300 ml of biodiesel is reported.
The cold soak filter test is supposed to be independent of the type of biodiesel feedstock. However, there is no adequate documentation on cold soak filtration test results from biodiesel that gels at higher temperatures.